Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV
Por um escritor misterioso
Descrição
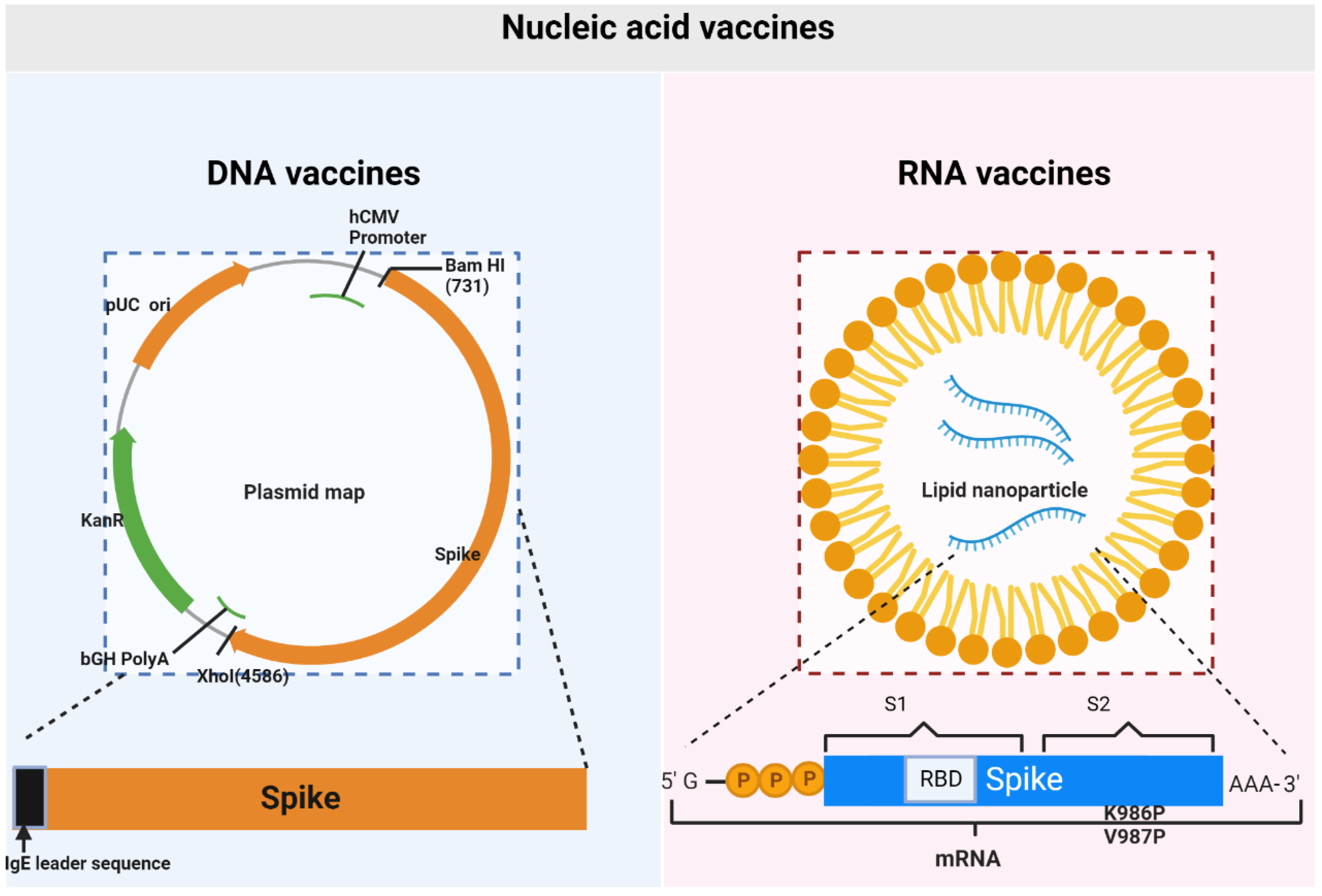
Vaccines, Free Full-Text

Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: A randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA Study) - eClinicalMedicine

COVID-19 Vaccine Frontrunners and Their Nanotechnology Design

A brief review on DNA vaccines in the era of COVID-19

Current Status of COVID-19 Vaccine Development: Focusing on Antigen Design and Clinical Trials on Later Stages

Potential SARS-CoV-2 & COVID-19 Vaccines
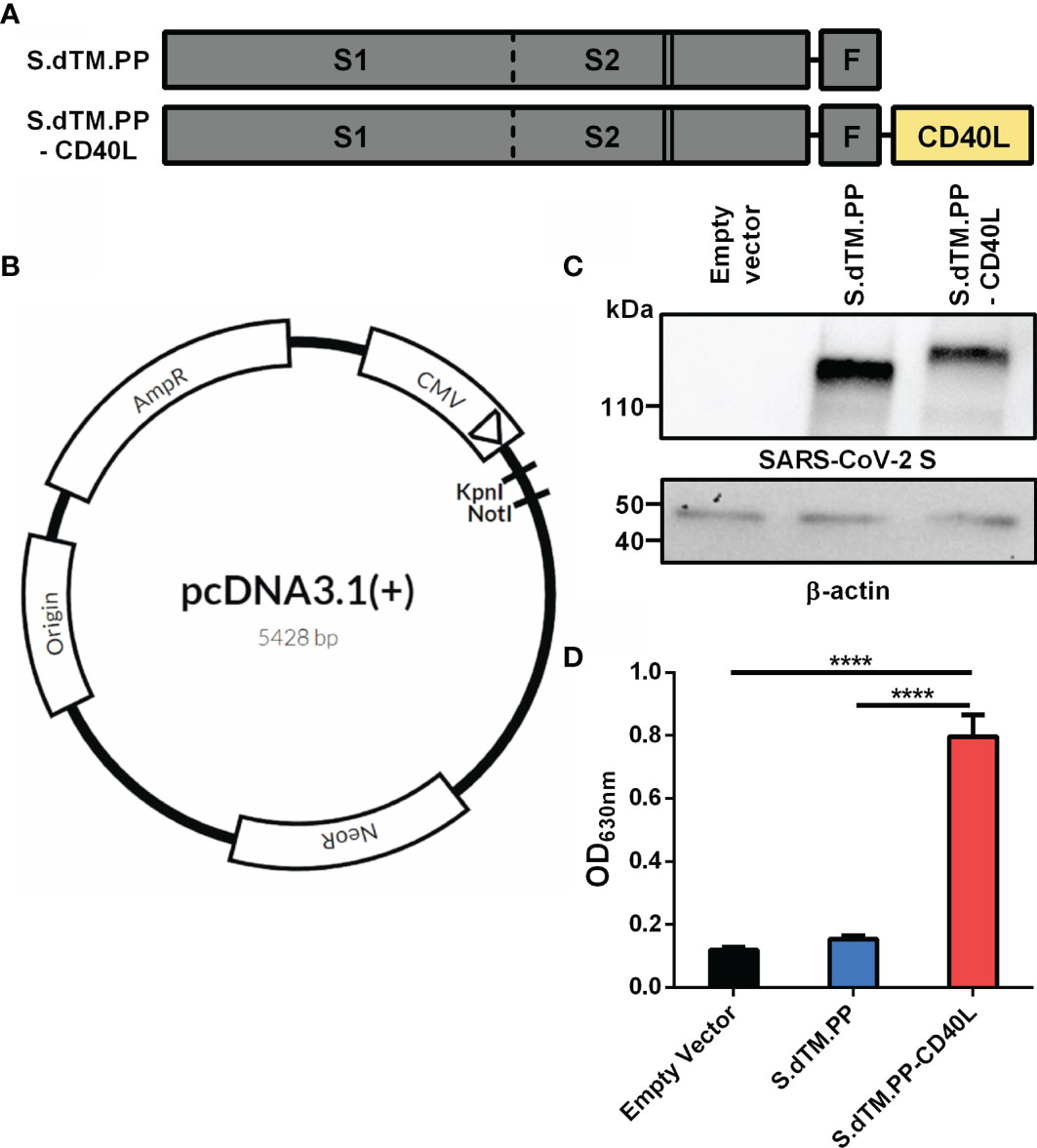
Frontiers DNA Based Vaccine Expressing SARS-CoV-2 Spike-CD40L Fusion Protein Confers Protection Against Challenge in a Syrian Hamster Model
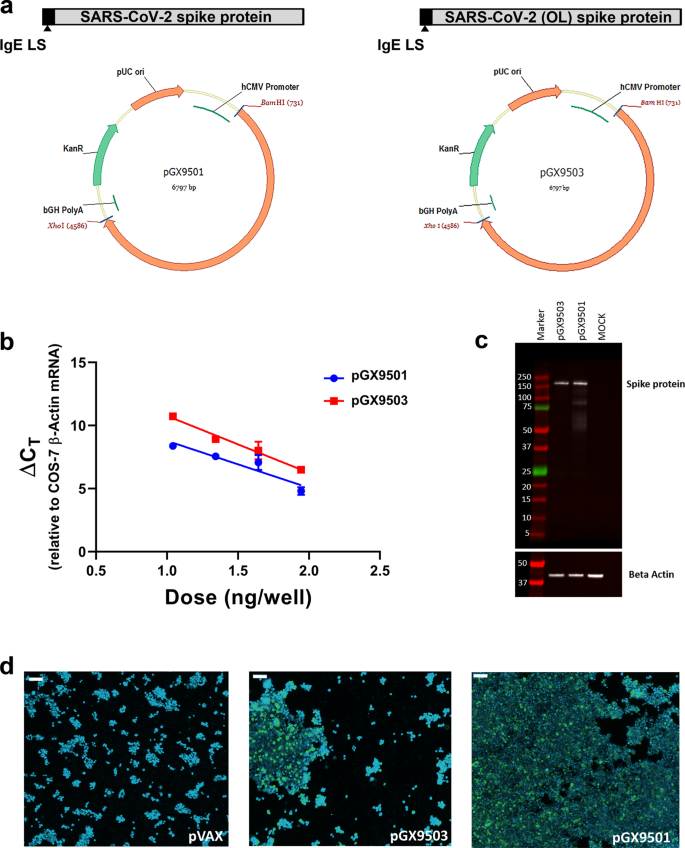
Immunogenicity of a DNA vaccine candidate for COVID-19
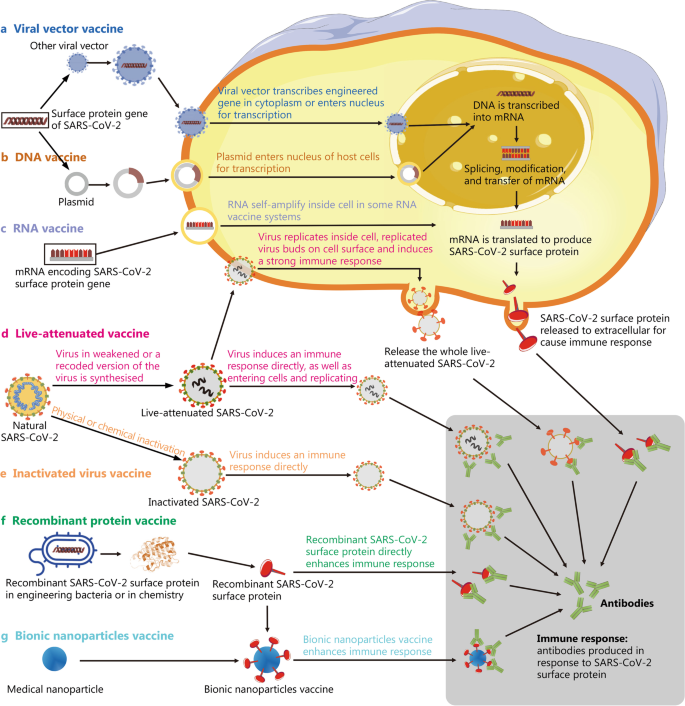
Advances in the design and development of SARS-CoV-2 vaccines, Military Medical Research
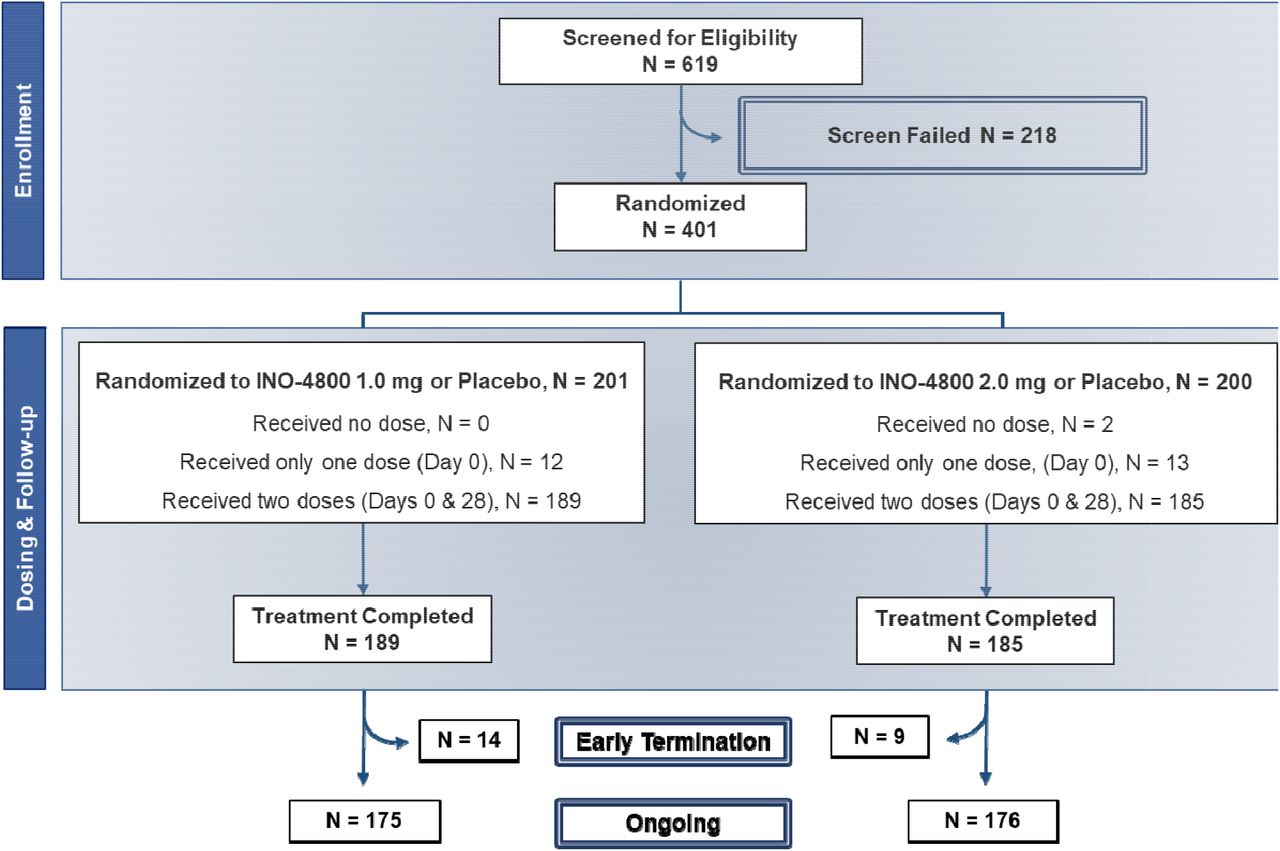
Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure

Intradermal DNA vaccine delivery using vacuum-controlled, needle-free electroporation: Molecular Therapy - Nucleic Acids

Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial - eClinicalMedicine
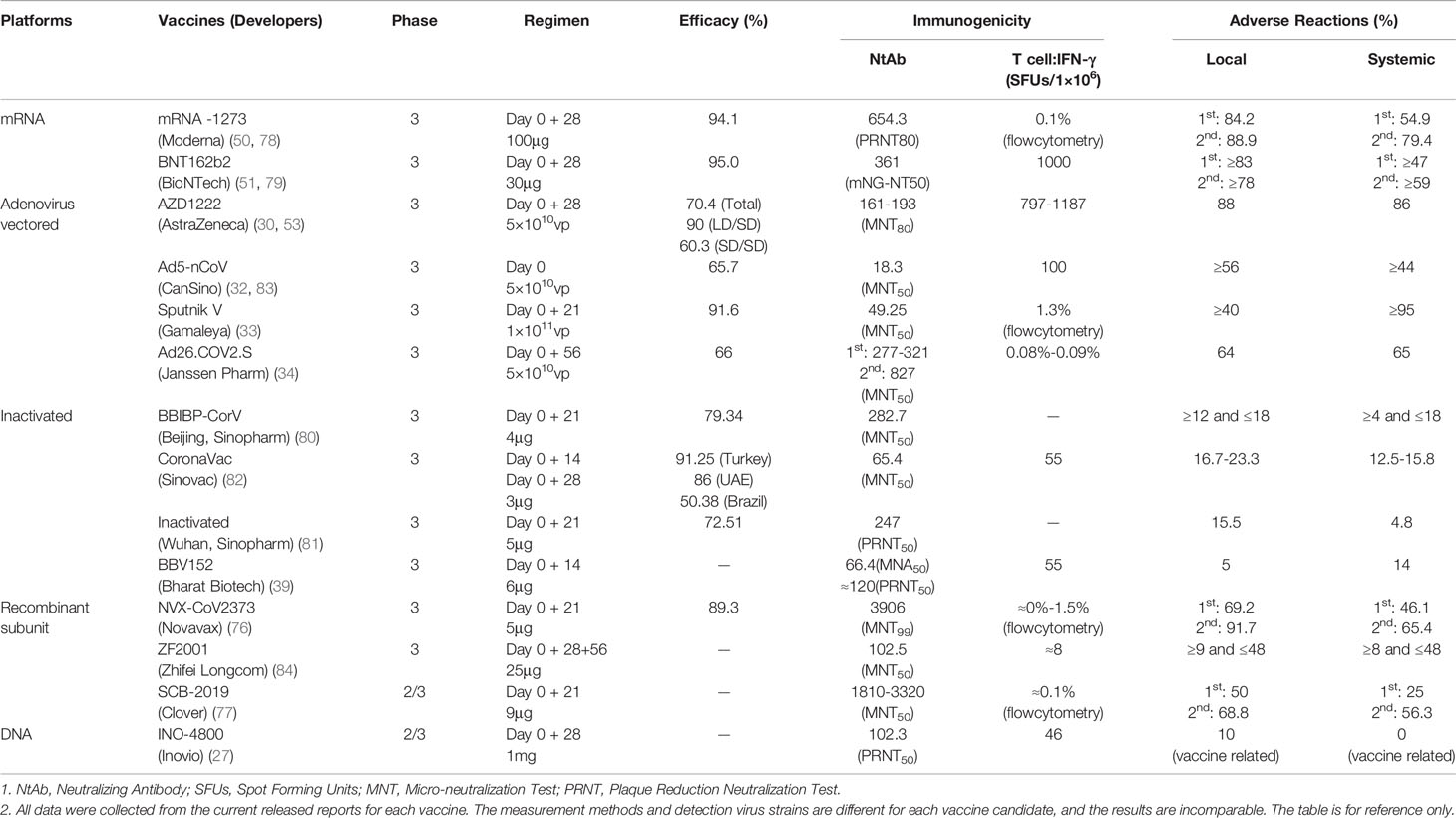
Frontiers COVID-19 Vaccines: Current Understanding on Immunogenicity, Safety, and Further Considerations

Phase 1 U.S. Trial of COVID-19 DNA Vaccine Enrollment Complete

EX-99.1
de
por adulto (o preço varia de acordo com o tamanho do grupo)