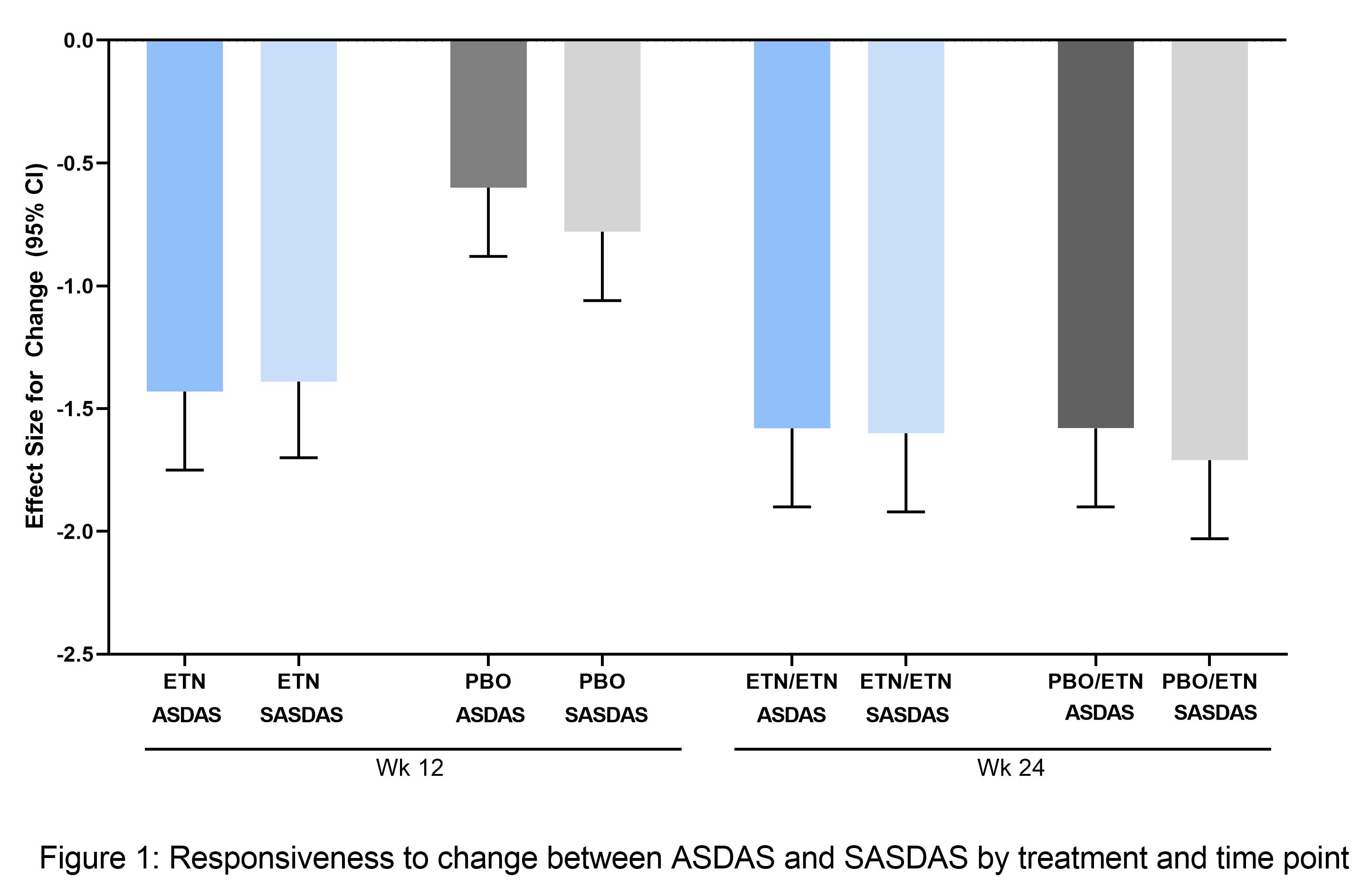
A. Mean ASDAS and B. mean BASDAI to week 96. Safety set (N = 89).
Por um escritor misterioso
Descrição
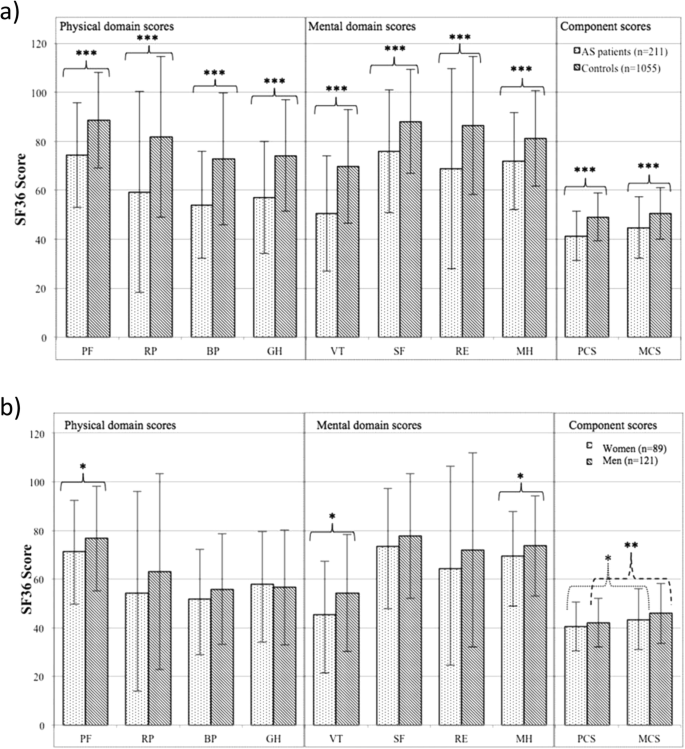
Factors related to health-related quality of life in ankylosing spondylitis, overall and stratified by sex, Arthritis Research & Therapy

These highlights do not include all the information needed to use CYLTEZO safely and effectively. See full prescribing information for CYLTEZO. CYLTEZO® (adalimumab-adbm) injection, for subcutaneous use Initial U.S. Approval: 2017 CYLTEZO (

Comparison of non-radiographic axial spondyloarthritis and ankylosing spondylitis patients – baseline characteristics, treatment adherence, and development of clinical variables during three years of anti-TNF therapy in clinical practice – topic of
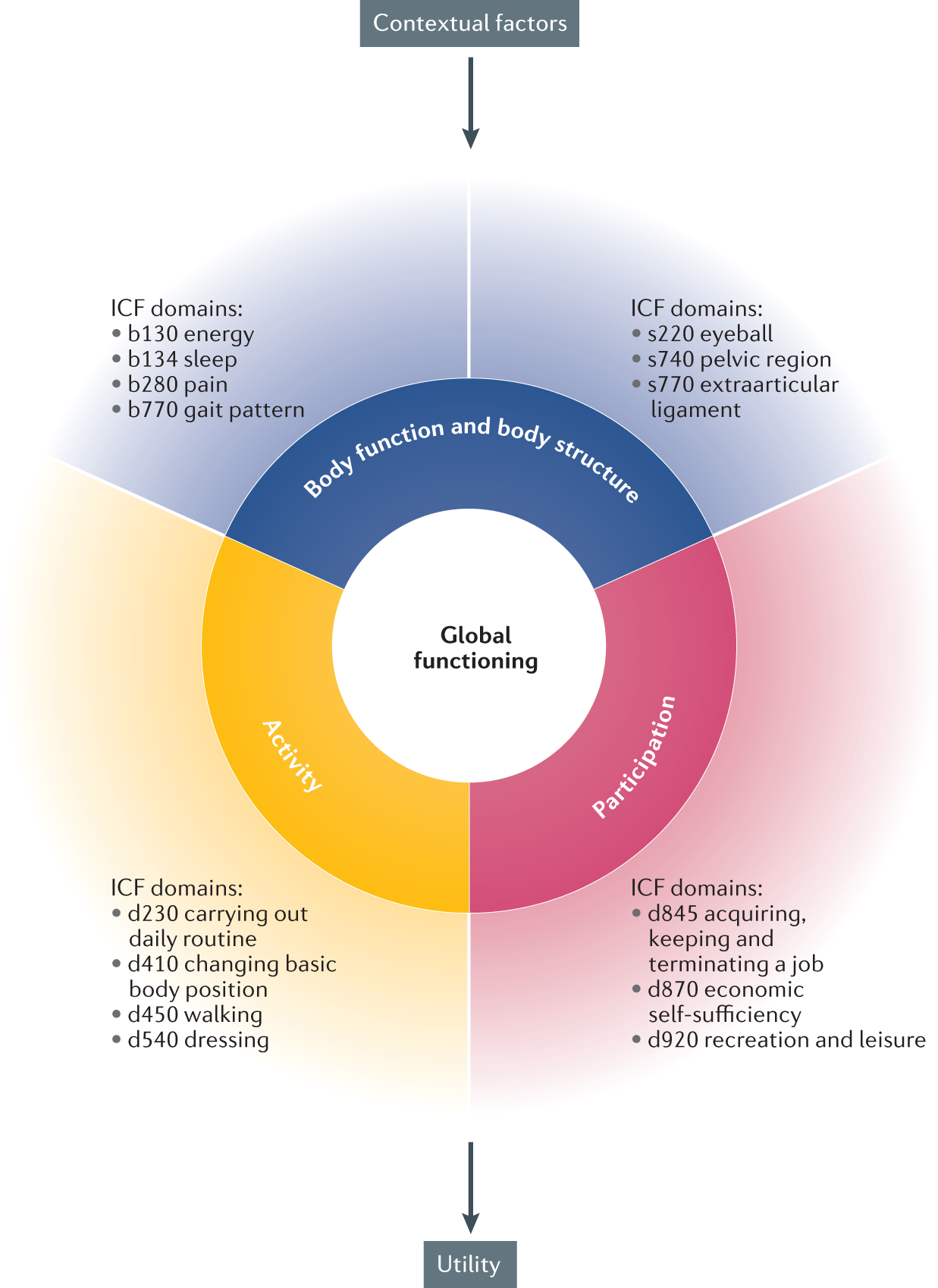
Treat-to-target in axial spondyloarthritis — what about physical function and activity?

TNF Inhibitor Therapy, Ankylosing Spondylitis
/index.php/cjht/article/downlo

Full article: POSTER PRESENTATIONS

THU0379 REDUCTION OF ANTERIOR UVEITIS FLARES IN PATIENTS WITH AXIAL SPONDYLOARTHRITIS FOLLOWING ONE YEAR OF TREATMENT WITH CERTOLIZUMAB PEGOL: 48-WEEK INTERIM RESULTS FROM A 96-WEEK OPEN-LABEL STUDY
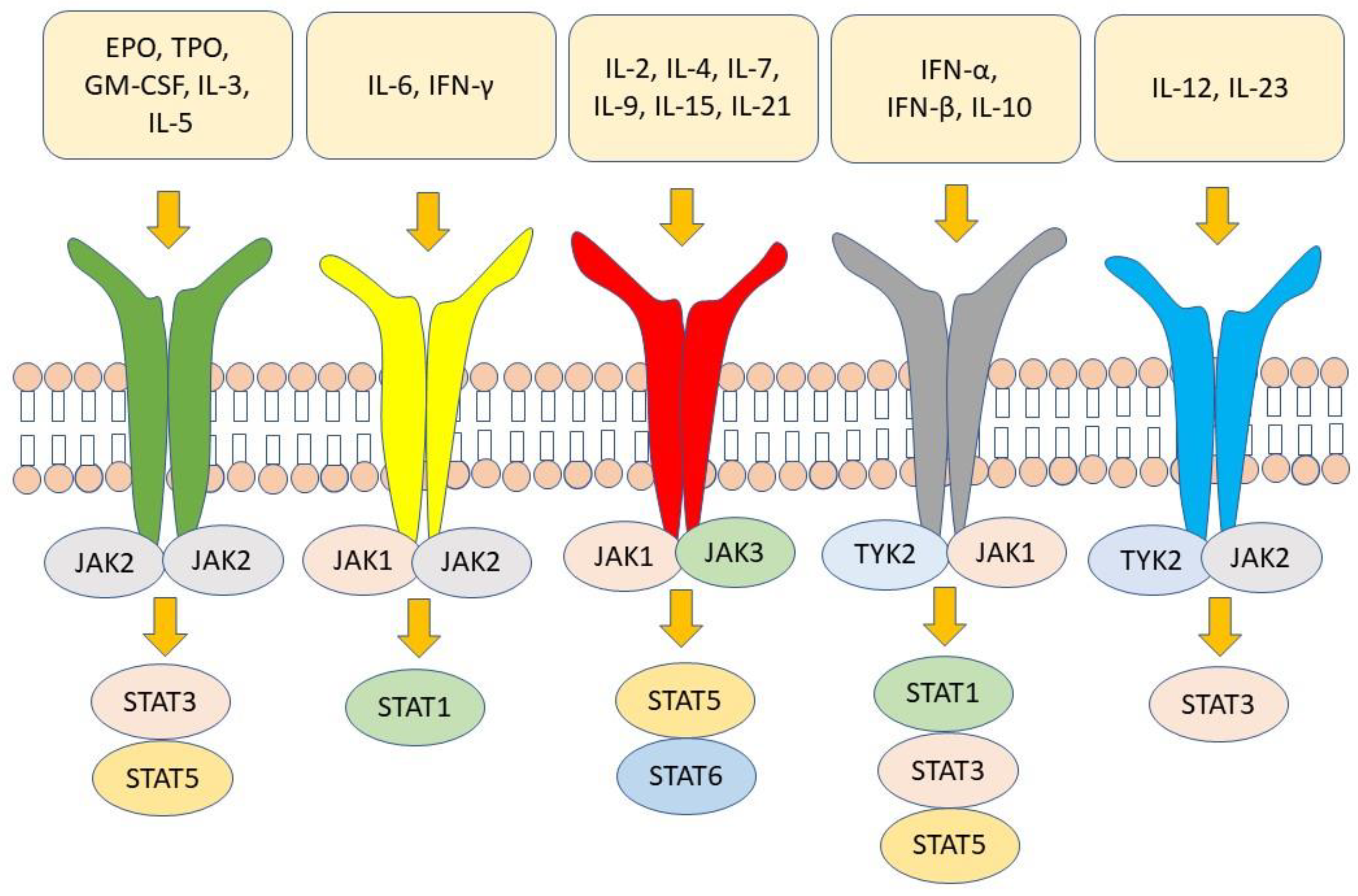
IJMS, Free Full-Text

Reduction of anterior uveitis flares in patients with axial spondyloarthritis on certolizumab pegol treatment: final 2-year results from the multicenter phase IV C-VIEW study - Irene E. van der Horst-Bruinsma, Rianne E.

Recommendations by the Spanish Society of Rheumatology on the Use of Biological Therapies in Axial Spondyloarthritis - ScienceDirect

Mean (A) ASDAS and (B) BASDAI up to Week 48. Safety Set (N=89).
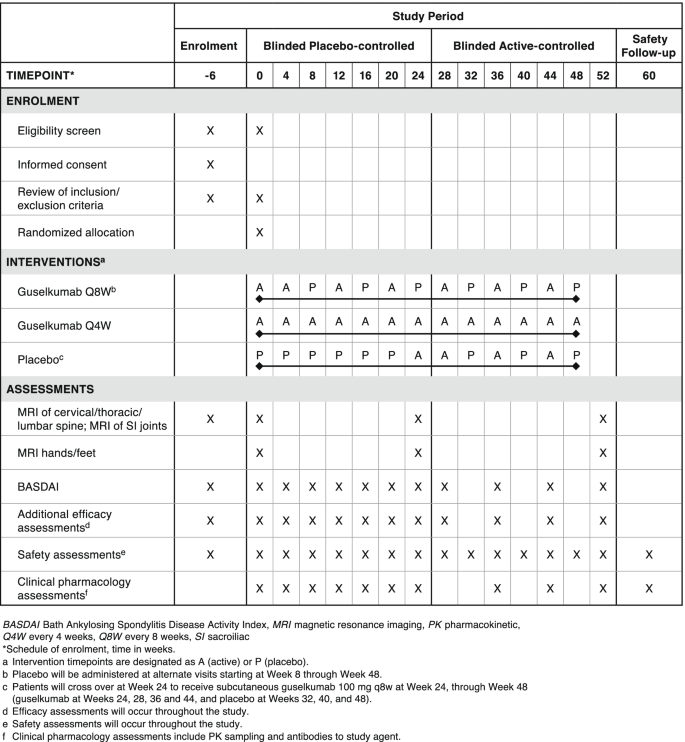
Efficacy and safety of guselkumab in biologic-naïve patients with active axial psoriatic arthritis: study protocol for STAR, a phase 4, randomized, double-blinded, placebo-controlled trial, Trials
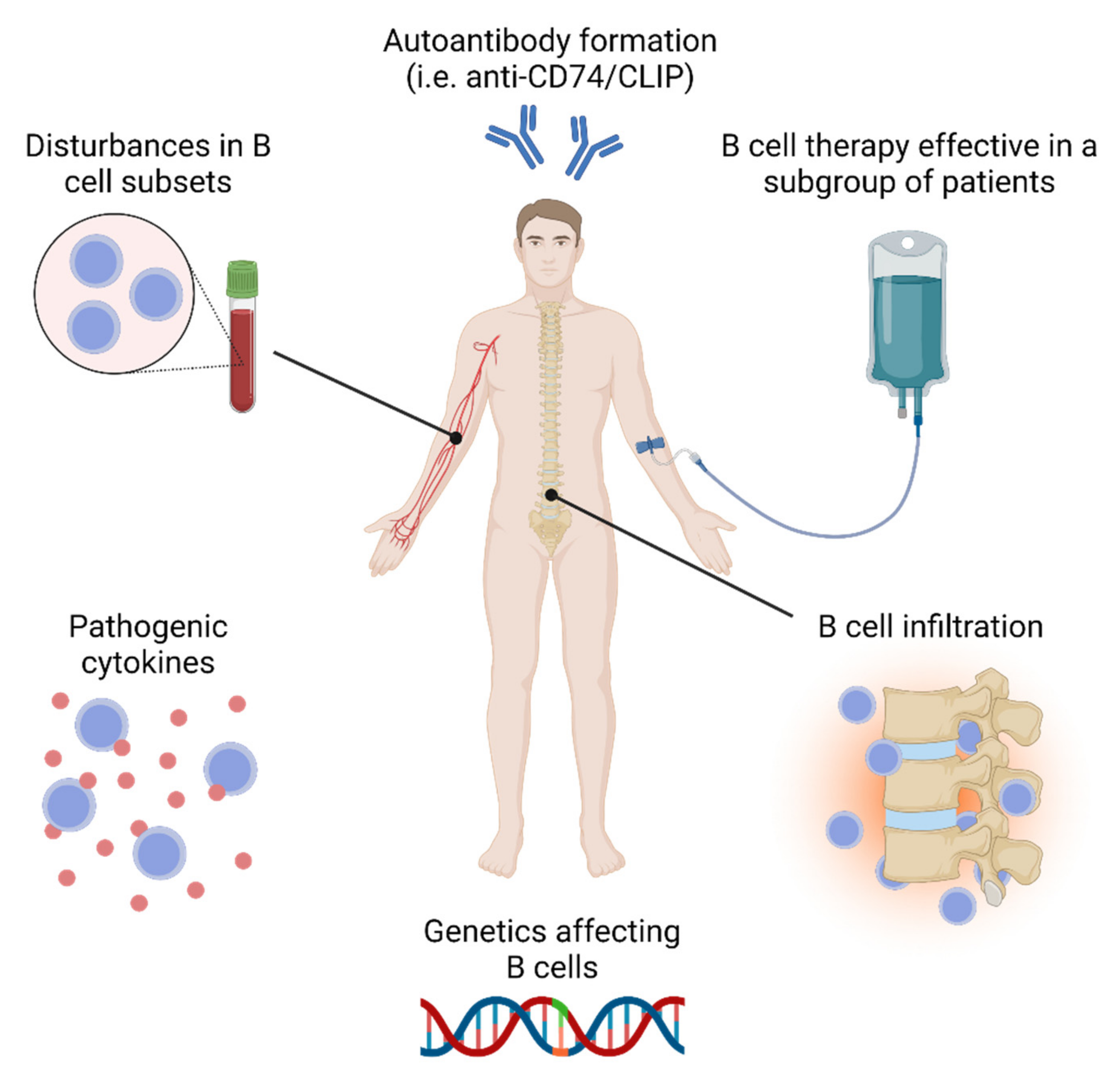
IJMS, Free Full-Text

Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial - The Lancet
de
por adulto (o preço varia de acordo com o tamanho do grupo)