FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Descrição
CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

Breakthrough Therapy Designation
FDA Approves First Drug to Treat Agitation Symptoms Associated with Dementia due to Alzheimer's Disease - USPTO
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

FDA Fast Track and Priority Review Programs

FDA approves supplemental new drug application for Rexulti to treat Alzheimer's agitation
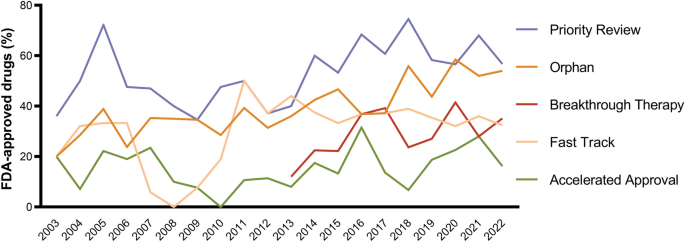
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy
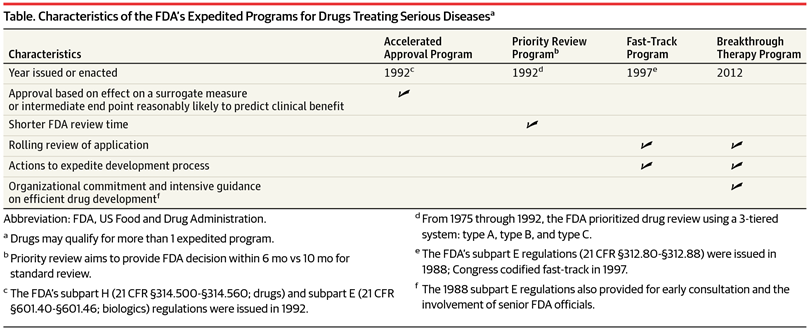
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB)

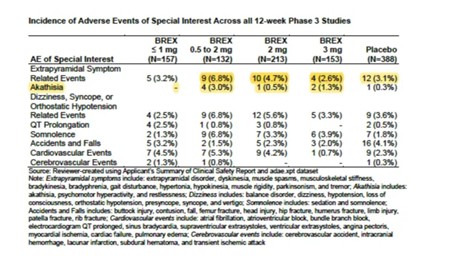
FDA rushes approval of dementia drug that quadruples risk of death
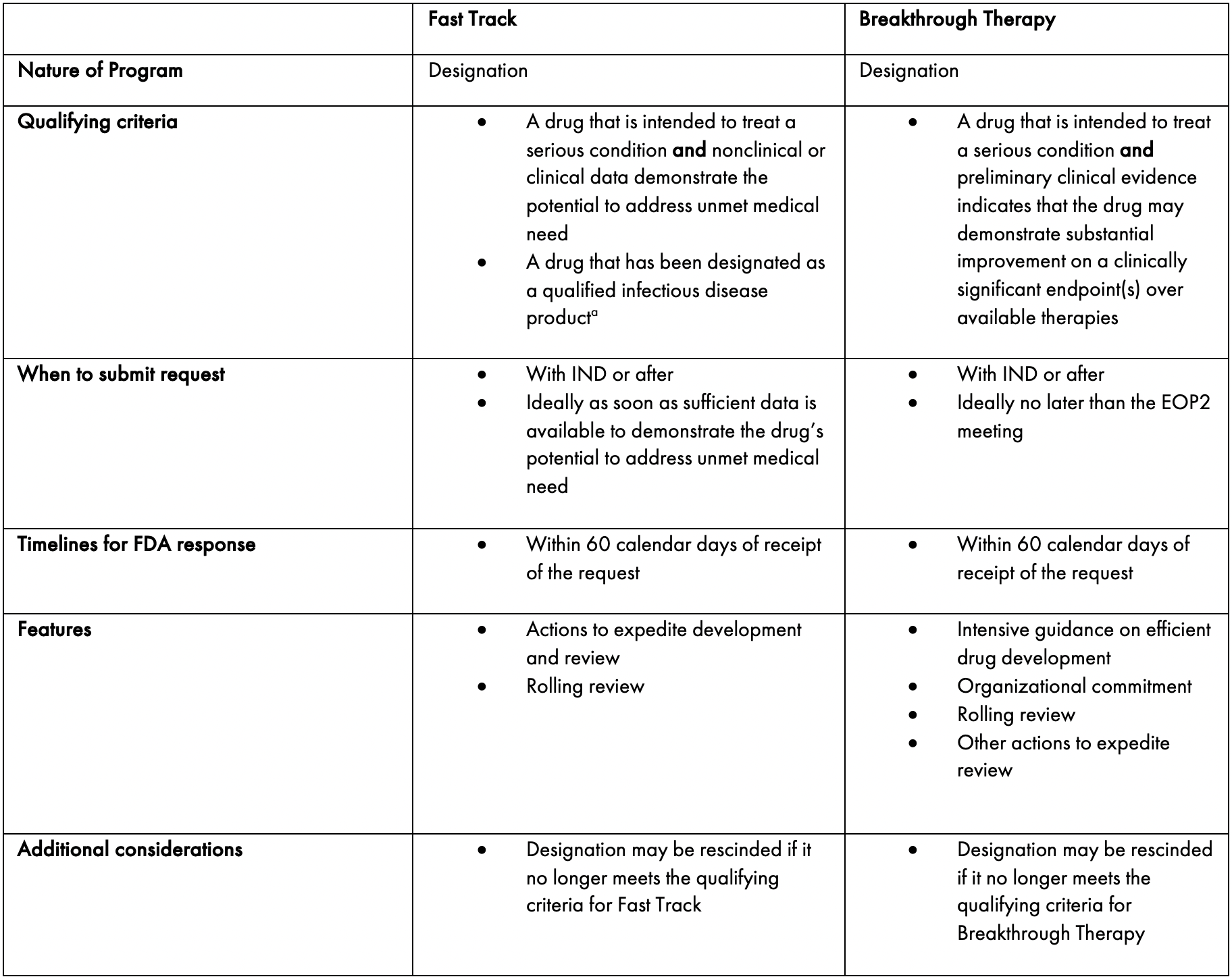
Fast Track Designation and Breakthrough Therapy Designation — Scendea
de
por adulto (o preço varia de acordo com o tamanho do grupo)