FDA allows Houston cancer doctor to resume drug trial
Por um escritor misterioso
Descrição
Federal regulators have lifted a partial hold on a clinical trial performed by Stanislaw
New drug targeting high-risk children's cancer is ready for trials - The Institute of Cancer Research, London

FDA gives controversial doc green light to restart work

FDA issues warning to controversial Houston cancer doctor
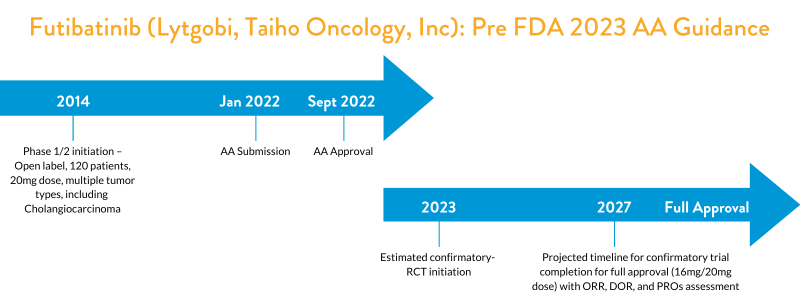
Accelerated Approval for Anticancer Products

Drug factory' implants eliminate ovarian, colorectal cancer in mice, Rice News, News and Media Relations

Brian_Wice

UTHealth Houston

Shubham Pant MD Anderson Cancer Center

Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study - The Lancet Oncology

FDA panel: Benefit of 'highly anticipated' lung cancer drug can't be interpreted reliably
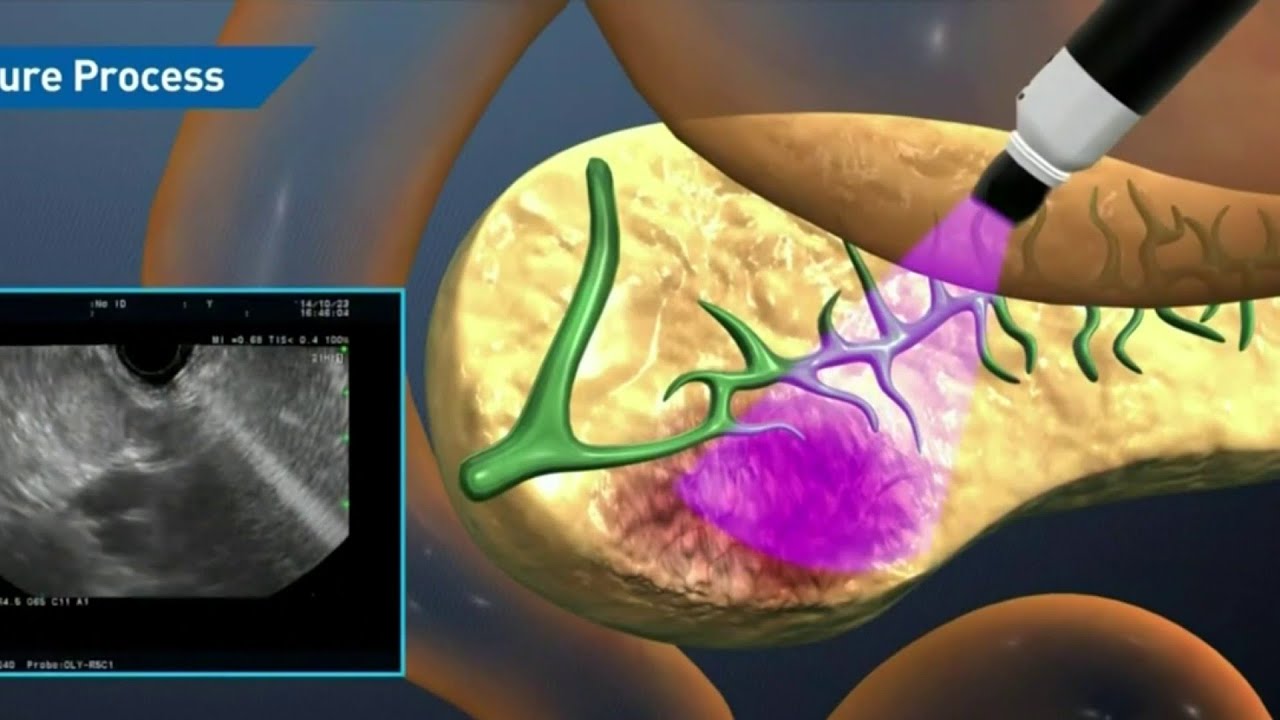
Houston doctor discovers way to treat pancreatic cancer during clinical trial

Sen. Sanders pushes NIH to rein in drug prices
FDA clears MD Anderson to test skin cancer treatment on humans

Texas Medical Board sanctions controversial cancer doctor Burzynski
de
por adulto (o preço varia de acordo com o tamanho do grupo)







