Designing an EDC System to Work for a CRA
Por um escritor misterioso
Descrição

Designing an EDC System to Work for a CRA

CTMS eTMF and EDC for Clinical Investigations

Consider EDC For Designing and building Clinical Trials
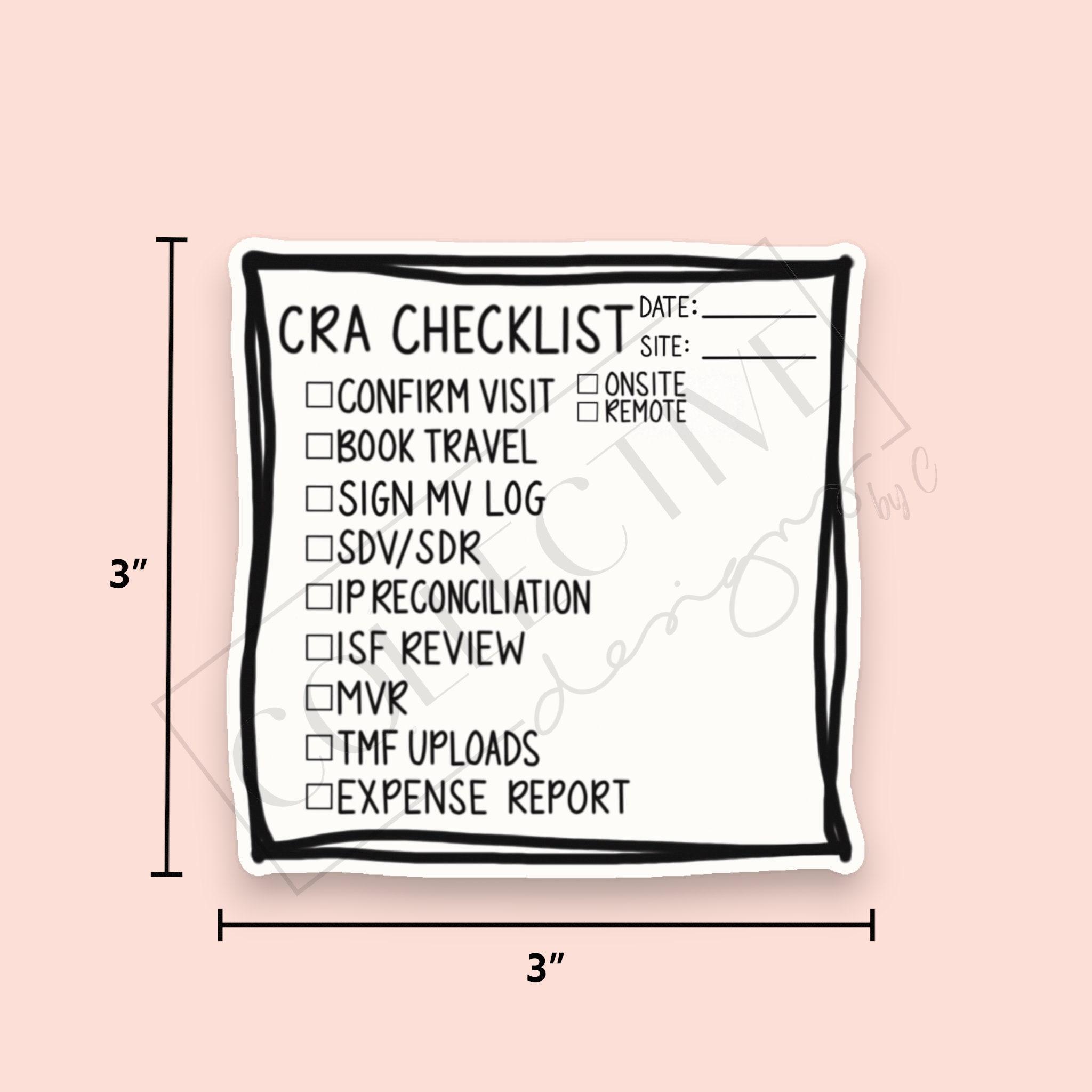
Clinical Research Post-it Note CRA Checklist Clinical
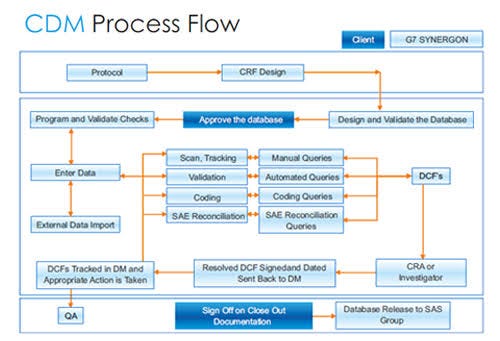
CLINICAL DATA MANAGEMENT — BASICS FOR FRESHERS
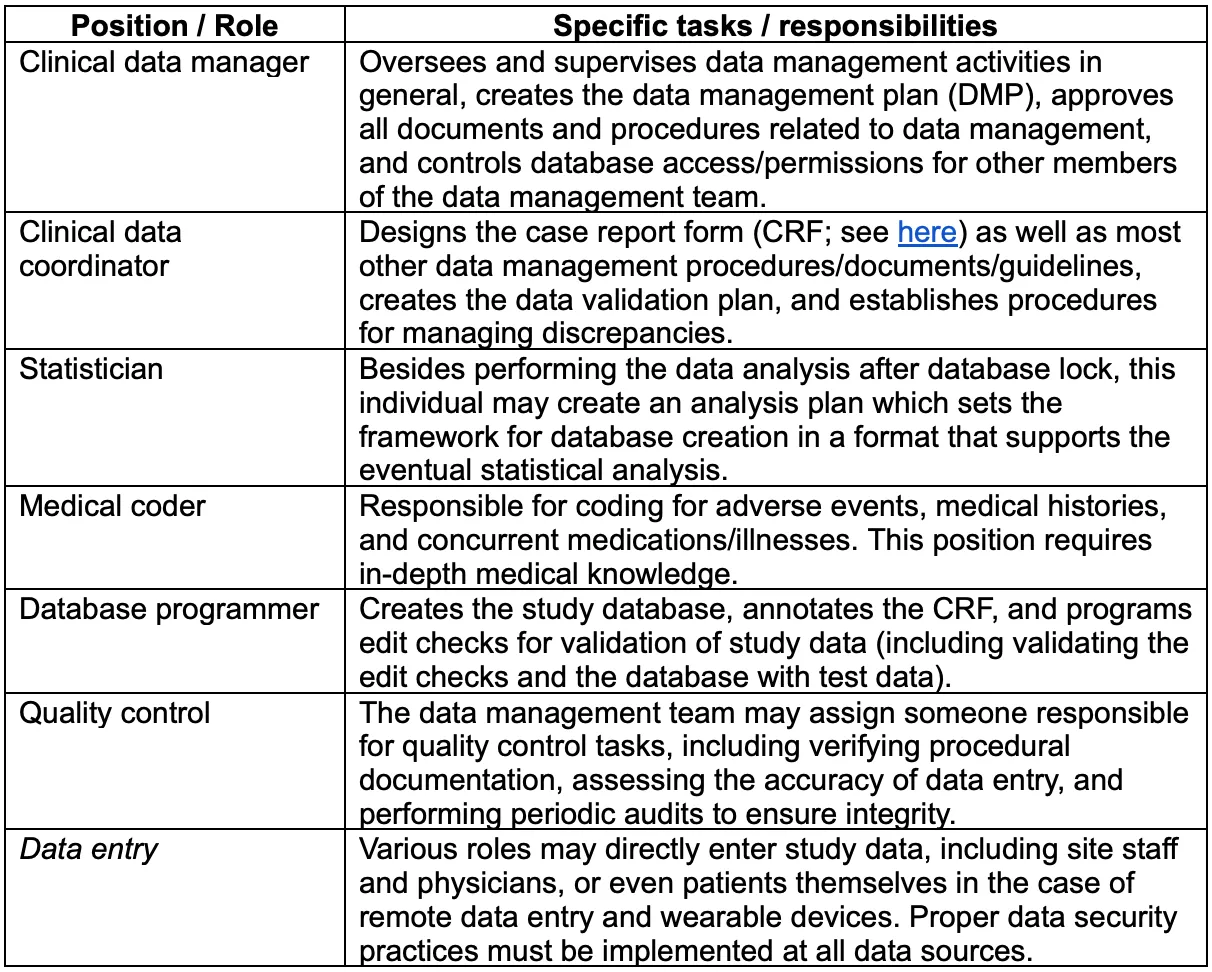
Clinical Trial Data Management

Cyrus Theuri, RN, MPH, CCRP on LinkedIn: #school #share #canada

Designing an EDC System to Work For a CRA - Xtalks

Rave Companion: Capture EHR Data Into Rave EDC In A Few Clicks
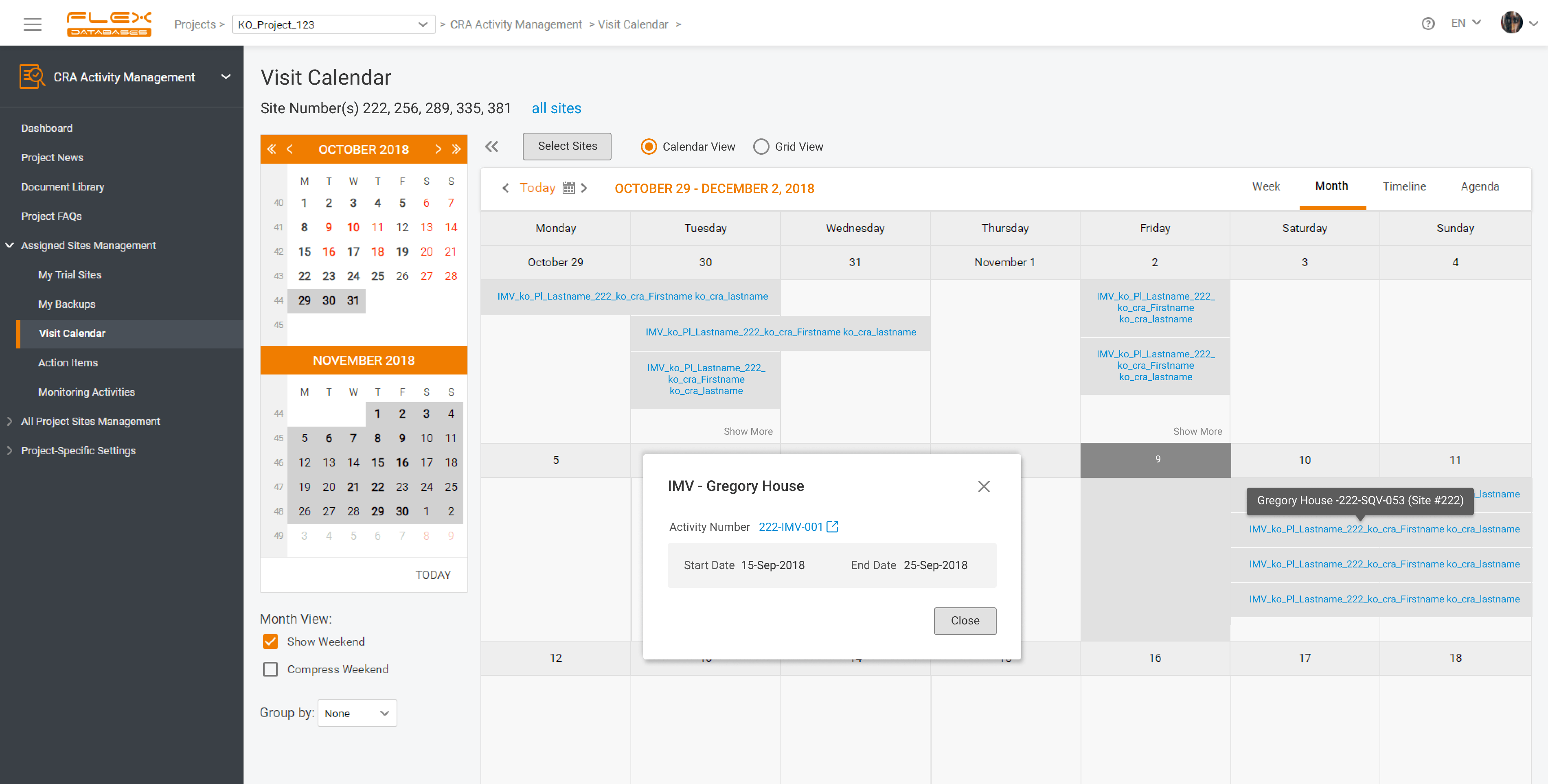
CTMS (Clinical Trial Management System) - Flex Databases
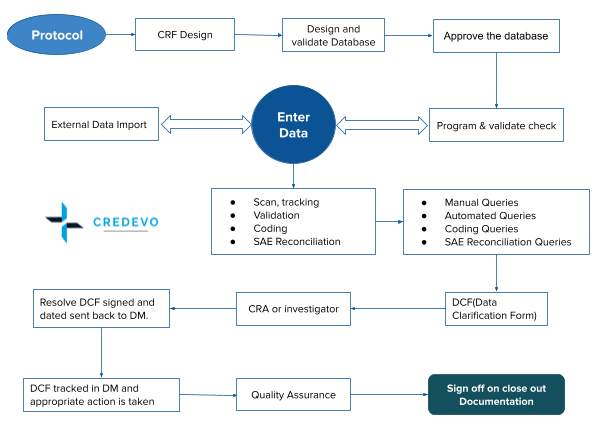
Data Management In Clinical Trials: Top 5 Important Aspects
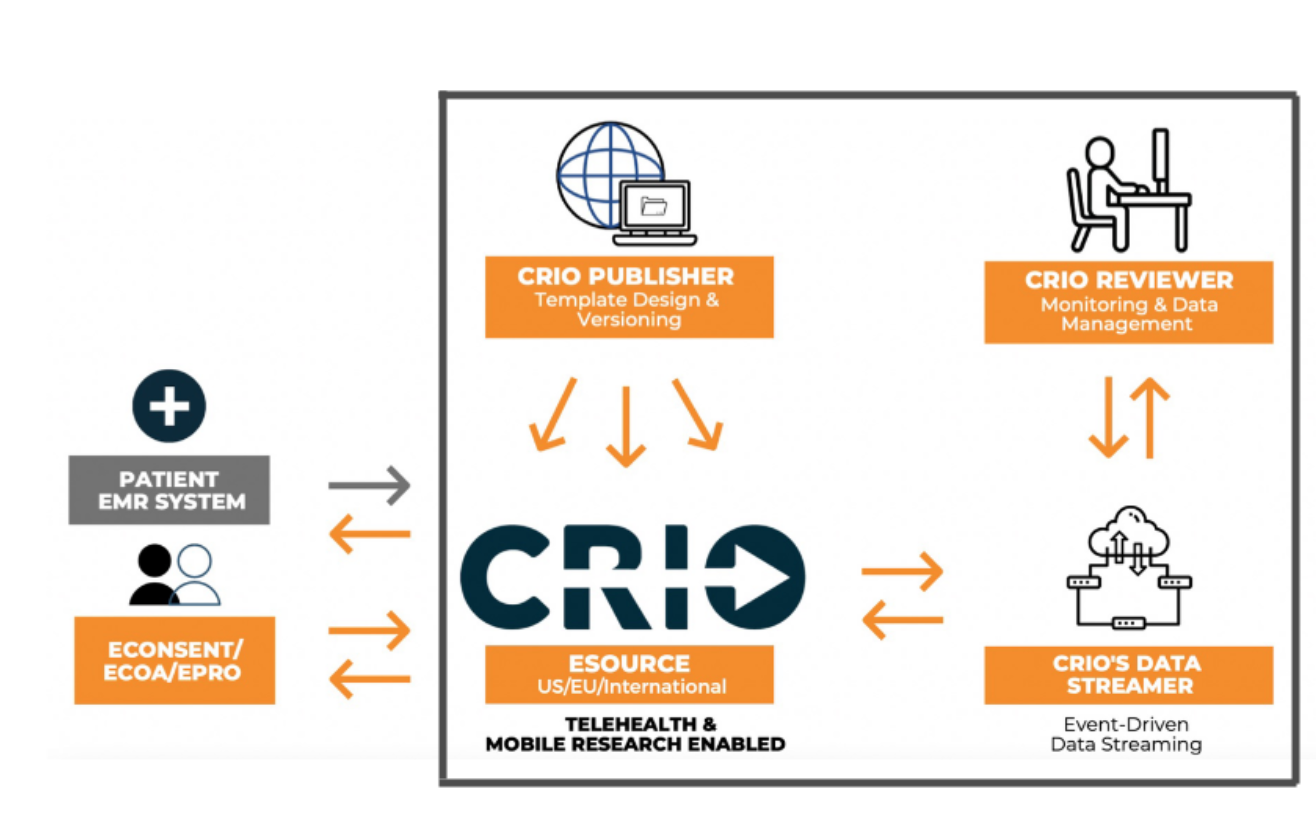
CRIO's integrated eSource-EDC Model is a Game Changer - CRIO

Clinical Research Associate Interview Questions - Gratisol Life
de
por adulto (o preço varia de acordo com o tamanho do grupo)







