Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Descrição
Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.
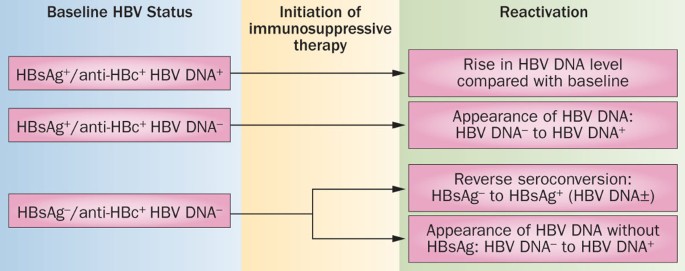
Management of patients with hepatitis B who require
Annalee Armstrong - Journalist Profile - Intelligent Relations
LA Weekend: Haunted Hayride; Jack-O'-Lantern Walk; Pull-A-Plane

IHEP (International Hepatology Education Program)

Materials about hepatitis B

Assembly Bio breaks off hepatitis B deal with Antios Therapeutics

Biotech Fierce Biotech

IHEP (International Hepatology Education Program) sur LinkedIn
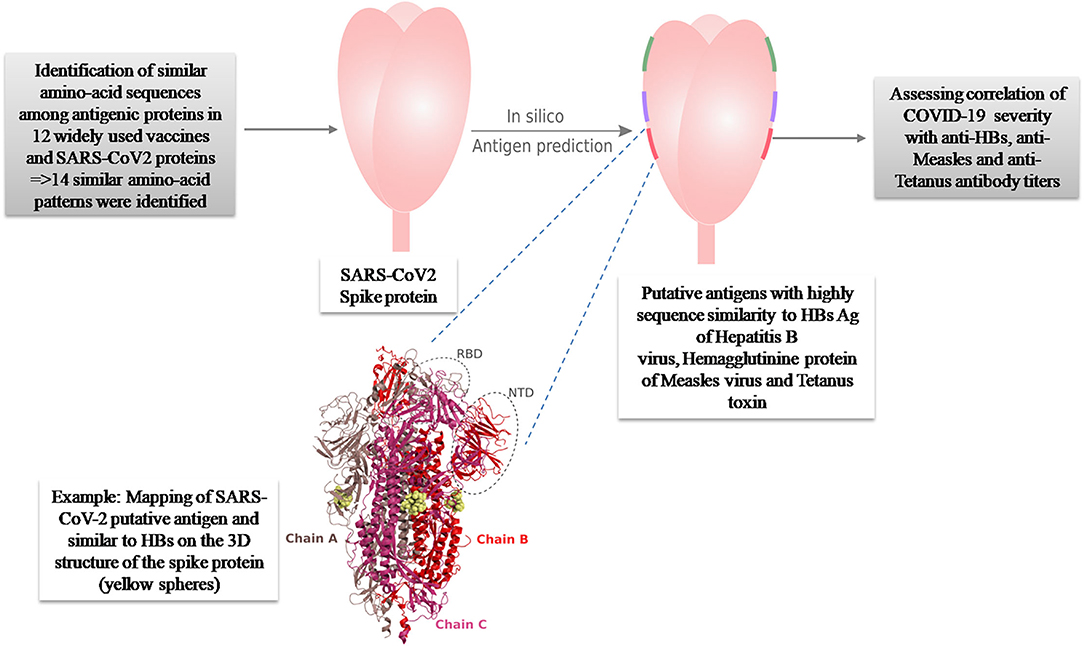
Frontiers Presumed Protective Role for Anti-Hepatitis B Virus

Annalee Armstrong - Journalist Profile - Intelligent Relations

AusperBio Announces FDA Clearance of IND Application of AHB-137 in

Landon Loving on LinkedIn: Fierce Biotech Fundraising Tracker '23

Robust hepatitis B vaccine-reactive T cell responses in failed
de
por adulto (o preço varia de acordo com o tamanho do grupo)





