ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial on the Treatment of Opioid Use Disorder (OUD)
Por um escritor misterioso
Descrição
ANANDA Scientific Inc., (a biotech pharma company) today announced approval by the U.S. Food and Drug Administration (FDA) of the Investigational New

ANANDA Scientific Announces FDA Approval of the IND for a Clinical Trial exploring treatment of Social Anxiety Disorder (SAD)
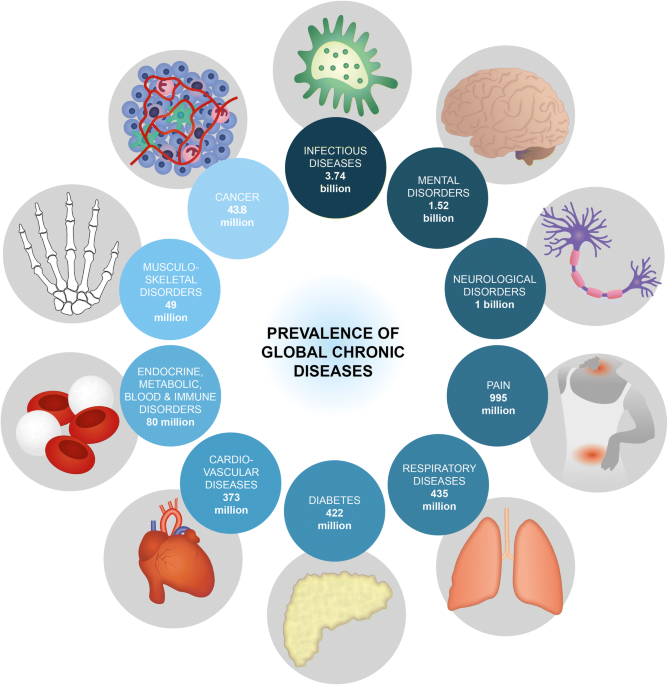
Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases

Ananda Scientific (@AnandaScience) / X

Ketamine, Esketamine, and A New Generation of Antidepressants

A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions

FDA approves IND for Ananda Scientific's PTSD treatment

Opioid Use Disorder Diagnosis and Management

The Evidence Supporting Treatment for Opioid Use Disorder (OUD)

ANANDA Scientific and David Geffen School of Medicine UCLA Announce Clinical Trial Utilizing Liquid StructureTM Cannabidiol (CBD) for the Treatment of Opioid Use Disorder (OUD) - 뉴스와이어

Ananda Scientific (@AnandaScience) / X

FDA-approved medications for OUD, with typical dosing paradigms for
de
por adulto (o preço varia de acordo com o tamanho do grupo)







